Hospital Trust first in UK to implant novel device to treat acid reflux using robotic surgery
University Hospital Southampton (UHS) has become the first NHS trust in the UK to implant a novel device to treat patients with severe acid reflux disease using robotic surgery.
Known as gastro-oesophageal reflux disease (GORD), the condition occurs when contents from the stomach flows back into the oesophagus – the long tube that carries food from the throat to the stomach.
It happens when the muscular valve – the lower oesophageal sphincter – at the bottom of the oesophagus becomes weakened because it has moved too close to the diaphragm or even into the chest which affects its function to allow food in and stop acid leaking out.
This can result in a range of symptoms including heartburn, regurgitation, difficulty swallowing, bloating, excessive salivation, coughing, nausea and a hoarse voice, as well as teeth and gum damage, nutritional problems, and sleep impairment.
The new “revolutionary” device, RefluxStop, is fixed to the upper part of the stomach wall and blocks movement of the lower oesophageal sphincter to hold it in its original, natural position and restore normal anatomy and function.
Made out of medical grade rounded solid silicone, the implant measures around 25mm – smaller than a ping pong ball – and is fitted via robotic-assisted laparoscopic (keyhole) surgery as a day case, with patients in theatre for less than two hours.
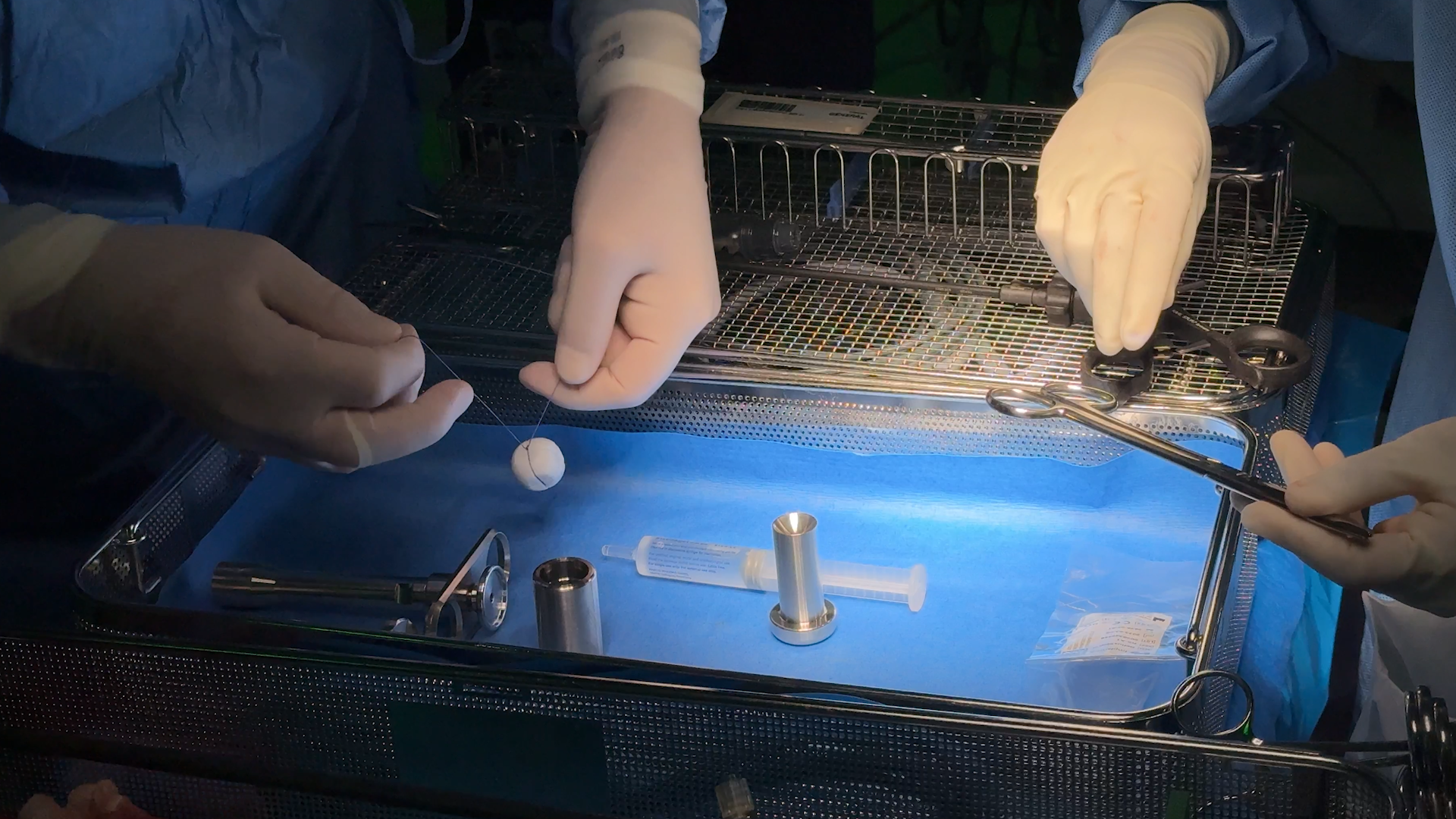
Approximately 20 per cent of the adult UK population have problems with severe acid reflux or GORD and UHS treats around 50 patients every year with the condition.
It is the second site in the UK to implant RefluxStop after Imperial College London but the first to do so using robotic surgery.
The disease can also lead to further complications such as oesophageal ulcers, a scarred and narrow oesophagus, Barrett’s oesophagus – changes to the cells in the lining of the oesophagus – and oesophageal cancer.
It is estimated that one in every 10 to 20 people with Barrett’s oesophagus will develop oesophageal cancer within 10 to 20 years.
The non-active implant is expected to last a lifetime and patients who receive the surgery should see an almost immediate improvement to their symptoms once they have fully recovered from the procedure.
Current standard treatments for GORD include a drug therapy called proton pump inhibitors (PPI) and a surgical procedure known as the fundoplication method, but these are not suitable for all patients and some continue to experience symptoms.
Danielle Harding, 30, recently became the first patient with GORD to undergo the procedure at UHS.
The mum of two, from Southampton, said: “I began suffering with severe acid reflux in 2022 and it has affected my life in so many ways – anything that involves eating or drinking has caused me so much anxiety, especially outside of my home.
“The signs are almost immediate with an intense pain in my chest as the reflux comes up the oesophagus and burns my throat, often resulting in me vomiting – it’s been horrendous."
Danielle was prescribed medication from her GP which initially stopped the symptoms with the hope that after one month the condition would settle down – but the symptoms returned immediately. An endoscopy showed that Danielle had significant damage to her oesophagus and that she would need to remain on a high dose of medication for life.
“The medication had side effects such as irritable bowel syndrome (IBS), which would cause me bad stomach pains that would stop me in my tracks and I wasn’t keen on being dependent on medication for life, particularly at such a young age,” she explained.
“We spoke about the fundoplication surgical option but that seemed quite invasive and also had potential side effects. I felt I had to choose between having GORD or having to live with side effects from medication or surgery.”
She added: “Then the team at the hospital offered me the option of RefluxStop, a non-active life-long implant that restores function – it seemed like a no-brainer. I had the surgery a little over a month ago and my symptoms have completely disappeared. I’m absolutely delighted and am so grateful to Fergus and the team at UHS, it has changed my life.”

Fergus Noble, consultant general and oesophagogastric surgeon at UHS, said: “We are delighted to be the first site in the south to offer this procedure to patients suffering with this chronic and debilitating condition and the first in the UK to implant it using robotic surgery.
“GORD can have such a severe impact on a person’s daily life as not only do they suffer with the physical impact whenever they swallow and eat, but also the psychological impact of living with the condition.
“RefluxStop is revolutionary in that it is minimally invasive, restores the lower oesophageal valve to its natural position with no side effects and offers some patients a viable treatment option for the first time.”
James Byrne, consultant general and upper gastrointestinal surgeon at UHS, added: “This is a really positive development for patients with GORD as we know current treatments are not always suitable or successful.
“PPI therapy treats the symptoms of the condition and not the cause, meaning patients can still suffer with symptoms and acidity remains present, while fundoplication surgery involves wrapping the stomach around the bottom of the oesophagus to strengthen the sphincter valve.
“However, as it is not a valve and cannot open and close, patients often experience side effects after surgery such as difficulty swallowing and an inability to belch or vomit. We believe people having RefluxStop may have less frequent and severe side effects than other anti-reflux procedures and this is fantastic news for patients and clinicians.”
Dr. Peter Forsell, founder and CEO of Implantica and the inventor of the RefluxStop device, said: “I am excited to see RefluxStop become available to patients in the South of England and we are proud to support Fergus, James and their wider team at UHS.
“Evidence shows RefluxStop has a positive impact on both quality of life and the cost-efficiency of GORD treatment, so we are delighted to see it being rolled out further for patients.”