Service development and innovation (scientific computing)
Neurological physics
We have developed an ICP Reporter tool to generate clinical reports from intracranial pressure (ICP) monitoring data produced by the clinical ICP monitoring of a patient using the Raumedic data logger device. This software was built to improve the efficiency and consistency of creating reports from the recorded data, a process that Tony Birch had kicked off using MATLAB code that often needed to be tweaked to handle specifics within particular datasets or requirements. ICP Reporter was developed with input from neurological physics and provides improved consistency and automation for our clinical colleagues, and produces a useful report that can be interacted with in a web browser and exported to PDF files for long-term attachment to medical records.
MRI physics
We developed this software to enable users to load magnetic resonance spectroscopy (MRS) datasets into a database maintained by this software, and compare them to a chosen cohort based on certain characteristics (for example patient age or pathologies). Once the dataset has been selected for analysis, and the cohort selected for comparison, metabolite amplitudes are displayed and colour-coded to aid comparison against statistical values for the cohort. Version 1.0 was released into use in 2022, and version 1.1 is an essential update due for release this year (2024) to enable processing and reporting of preterm patients, as well as including flags to exclude specific data sets from cohort averaging.
Nuclear medicine physics
We have developed a number of inhouse image-based decision support tools in collaboration with our nuclear medicine physics (NM) colleagues. These tools were initially developed as part of a larger ongoing project to transfer all image processing from legacy systems to a new platform under our QMS. One such example is Renogram Processing, which has been in clinical use for a number of years, and we have now completed development of the second update, v1.2. This adds in fully-automated motion correction capabilities, as well as functionality to tweak the parameters to make improvements if necessary. We plan to deploy this functionality to our other nuclear medicine applications and are currently doing this for DMSA processing.
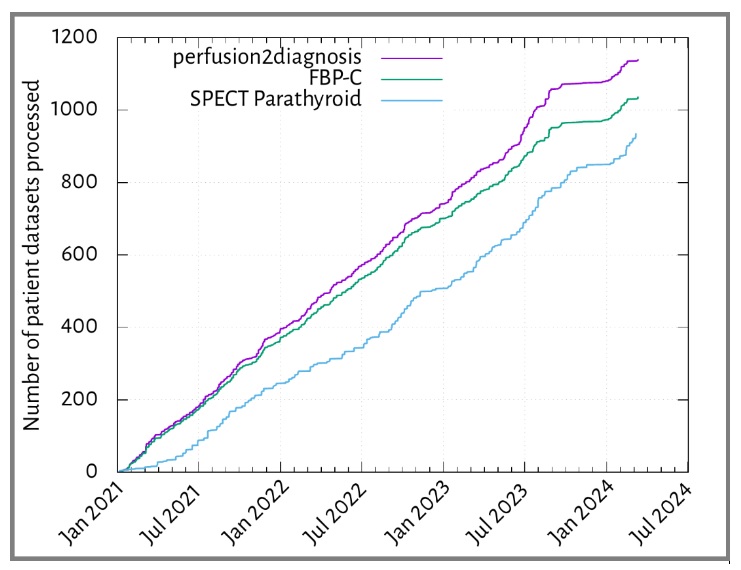
Tracking usage of nuclear medicine software already deployed
We have three services in clinical use by NM physics which involve data preparation by the users to create input datasets that are then automatically analysed by our software, that then provides suitable forms of output back to the originating system, formatted as DICOMs. These include a custom FBP image reconstruction tool that applies Chang's attenuation correction to the imaging data, the perfusion2diagnosis tool that analyses the reconstructed outputs and produces statistical output in a report to help guide clinical diagnoses, and SPECT parathyroid, which creates images of parathyroid activity from input data from clinical scans where hyperparathyroidism is indicated. The numbers of datasets processed from 2021 onwards are shown below.
Southampton Oxford retrieval team (SORT) PICU app
We have worked with members of the Southampton Oxford retrieval team and UHS pharmacy to produce an app to support paediatric intensive care unit (PICU) in retrievals. The app contains a comprehensive calculator which provides suggested doses of a wide range of appropriate drugs and infusions that may be required in such scenarios, based on the patient's biological sex, and either weight or age. It also contains a library of clinical guidelines in PDF format so they are available at the point of need, even in signal blackspots. We are aiming to release the app within the next few months as our first medical device 'on the market' via the UK Apple App Store, using the MDSG medical device software QMS.
Quality management system
The scientific computing team develop medical device software for routine clinical use. Unlike our non-clinical tools, these require additional considerations, for example around safety, quality and security, to ensure we are providing the highest quality care possible and complying with all applicable regulations. To meet this requirement we have built a QMS and, in collaboration with several teams in UHS Digital, formed the UHS medical device software group and have achieved external accreditation against ISO13485 from BSI. The scope of our accreditation is: "Design and development of bespoke medical device software for image and data analysis supporting patient diagnosis and patient treatment decisions for in-house applications, and medical device software for drug dosage calculation".
The following regulations and standards are included in the scope of the MDSG QMS:
- The UK Medical Device Regulations 2002
- ISO 13485:2016 (Medical Devices - Quality Management Systems - Requirements for regulatory purposes)
- ISO 14971:2019 (Medical devices - Application of risk management to medical devices)
- EN 62304:2015 (Medical Device Software - Software Life Cycle Processes)
- NHS Digital Clinical Safety Team Standards:
- DCB0160: Clinical Risk Management: its Application in the Deployment and Use of Health IT Systems
- DCB0129: Clinical Risk Management: its Application in the Manufacture of Health IT Systems
In addition, as we are passionate about building increasing levels of automation and deriving new insights from clinical data, we are actively reviewing available guidance and publications to help us develop a safe and effective framework for building clinical applications that utilise AI. In addition, we have a number of projects where we are currently investigating whether the addition of AI would improve outcomes, safety and/or efficiency, and we are using these as pilot projects to help us develop our own processes in line with currently-available best practice guidance.